Advanced Oxidation Processes (AOPs) represent a set of chemical treatment procedures designed to remove organic (and sometimes inorganic) materials in water and wastewater by oxidation through reactions with hydroxyl radicals. These processes are characterized by the generation of highly reactive species that can effectively degrade a wide range of pollutants, including those that are resistant to conventional treatment methods. The application of AOPs in water treatment has gained significant attention due to their ability to achieve high levels of contaminant removal and improve water quality.
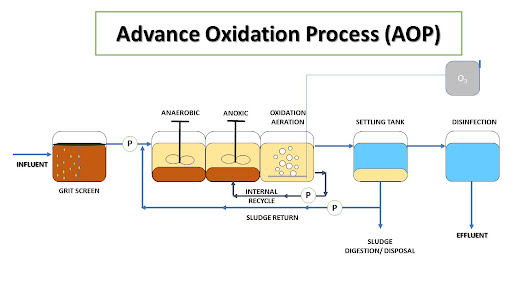
From an engineering perspective, AOPs involve the integration of various components and systems to facilitate the generation and application of reactive species. The key components of an AOP system typically include:
Oxidant Source: The primary oxidants used in AOPs include ozone, hydrogen peroxide, and ultraviolet light. These oxidants can be used individually or in combination to enhance the generation of hydroxyl radicals.
Reactor Design: The design of the reactor is crucial for the efficient generation and application of hydroxyl radicals. Common reactor configurations include batch reactors, continuous flow reactors, and photoreactors. The choice of reactor design depends on factors such as the type of oxidant used, the nature of the contaminants, and the desired treatment efficiency.
Hydraulic System: The hydraulic system ensures the proper mixing and contact between the oxidants and the water being treated. This system includes pumps, mixers, and flow control devices to maintain optimal conditions for the oxidation reactions.
Control and Monitoring Systems: Advanced control and monitoring systems are employed to regulate the operating parameters of the AOP system, such as oxidant dosage, reaction time, and temperature. These systems also monitor the performance of the treatment process and ensure compliance with regulatory standards.
The effectiveness of AOPs is primarily attributed to the generation of hydroxyl radicals, which are highly reactive and non-selective oxidizing agents. The generation of hydroxyl radicals can occur through various mechanisms, depending on the type of oxidant and the specific AOP employed. The following are some common methods for generating hydroxyl radicals:
Ozone-Based AOPs: In ozone-based AOPs, ozone is dissolved in water and decomposes to form hydroxyl radicals. The decomposition of ozone can be enhanced by the addition of hydrogen peroxide or by the application of ultraviolet light. The reactions involved in ozone-based AOPs include the interaction of ozone with water, hydrogen peroxide, or ultraviolet light to produce hydroxyl radicals and oxygen.
Hydrogen Peroxide-Based AOPs: Hydrogen peroxide can be activated by ultraviolet light or by transition metal catalysts (e.g., iron) to produce hydroxyl radicals. The reactions involved in hydrogen peroxide-based AOPs include the interaction of hydrogen peroxide with ultraviolet light or iron to produce hydroxyl radicals, hydroxide ions, and iron ions.
Photocatalysis: In photocatalytic AOPs, a semiconductor material (e.g., titanium dioxide) is used as a catalyst to generate hydroxyl radicals upon exposure to ultraviolet light. The reactions involved in photocatalysis include the excitation of the semiconductor material by ultraviolet light, leading to the generation of electron-hole pairs. The holes react with water to produce hydroxyl radicals, while the electrons react with oxygen to produce superoxide radicals.
The hydroxyl radicals generated through these mechanisms react with organic and inorganic contaminants in water, leading to their degradation and removal. The reactions between hydroxyl radicals and contaminants typically involve the abstraction of hydrogen atoms, addition to double bonds, and electron transfer processes.
Application in Water Treatment

AOPs have been widely applied in various water treatment applications, including the treatment of drinking water, industrial wastewater, and municipal wastewater. The following are some specific applications of AOPs in water treatment:
- 1. Drinking Water Treatment: AOPs are used to remove organic contaminants, such as pesticides, pharmaceuticals, and endocrine-disrupting compounds, from drinking water sources. The high reactivity of hydroxyl radicals ensures the effective degradation of these contaminants, resulting in improved water quality and safety.
- 2. Industrial Wastewater Treatment: AOPs are employed to treat industrial wastewater containing recalcitrant organic pollutants, such as dyes, solvents, and petrochemicals. The application of AOPs in industrial wastewater treatment can enhance the biodegradability of the wastewater, making it suitable for subsequent biological treatment processes.
- 3. Municipal Wastewater Treatment: AOPs are used as an advanced treatment step in municipal wastewater treatment plants to remove trace organic contaminants and improve the overall quality of the treated effluent. This is particularly important for wastewater reuse applications, where high-quality effluent is required for non-potable uses, such as irrigation and industrial processes.
Improvement of Water Treatment Processes
The integration of AOPs into water treatment processes offers several advantages that contribute to the overall improvement of water treatment efficiency and effectiveness. These advantages include:
Enhanced Contaminant Removal: AOPs can achieve high levels of contaminant removal, including the degradation of refractory organic pollutants that are resistant to conventional treatment methods. This results in improved water quality and reduced environmental impact.
Increased Biodegradability: The application of AOPs can increase the biodegradability of wastewater by breaking down complex organic molecules into simpler, more readily biodegradable compounds. This enhances the performance of subsequent biological treatment processes and reduces the overall treatment time and cost.
Reduction of Disinfection By-Products: AOPs can reduce the formation of disinfection by-products (DBPs) by degrading precursor compounds that contribute to DBP formation. This is particularly important in drinking water treatment, where DBPs pose a significant health risk.
Versatility and Flexibility: AOPs can be tailored to specific treatment needs by adjusting the type and dosage of oxidants, reaction conditions, and reactor design. This versatility allows for the effective treatment of a wide range of contaminants and water matrices.
Synergistic Effects: The combination of AOPs with other treatment processes, such as biological treatment, membrane filtration, and adsorption, can result in synergistic effects that enhance overall treatment efficiency. For example, the use of AOPs as a pre-treatment step can improve the performance of membrane filtration by reducing fouling and extending membrane life.




.gif)
