Chemical coagulation is a fundamental process in water treatment, employed to remove suspended particles, colloids, and other impurities from water. This process is essential for both drinking water and wastewater treatment, ensuring that water meets safety and quality standards. The process involves the addition of coagulants, which are chemicals that facilitate the aggregation of fine particles into larger flocs, making them easier to remove through subsequent treatment steps.
From an engineering perspective, the chemical coagulation process is meticulously designed to optimize the removal of impurities. The process typically involves several stages:
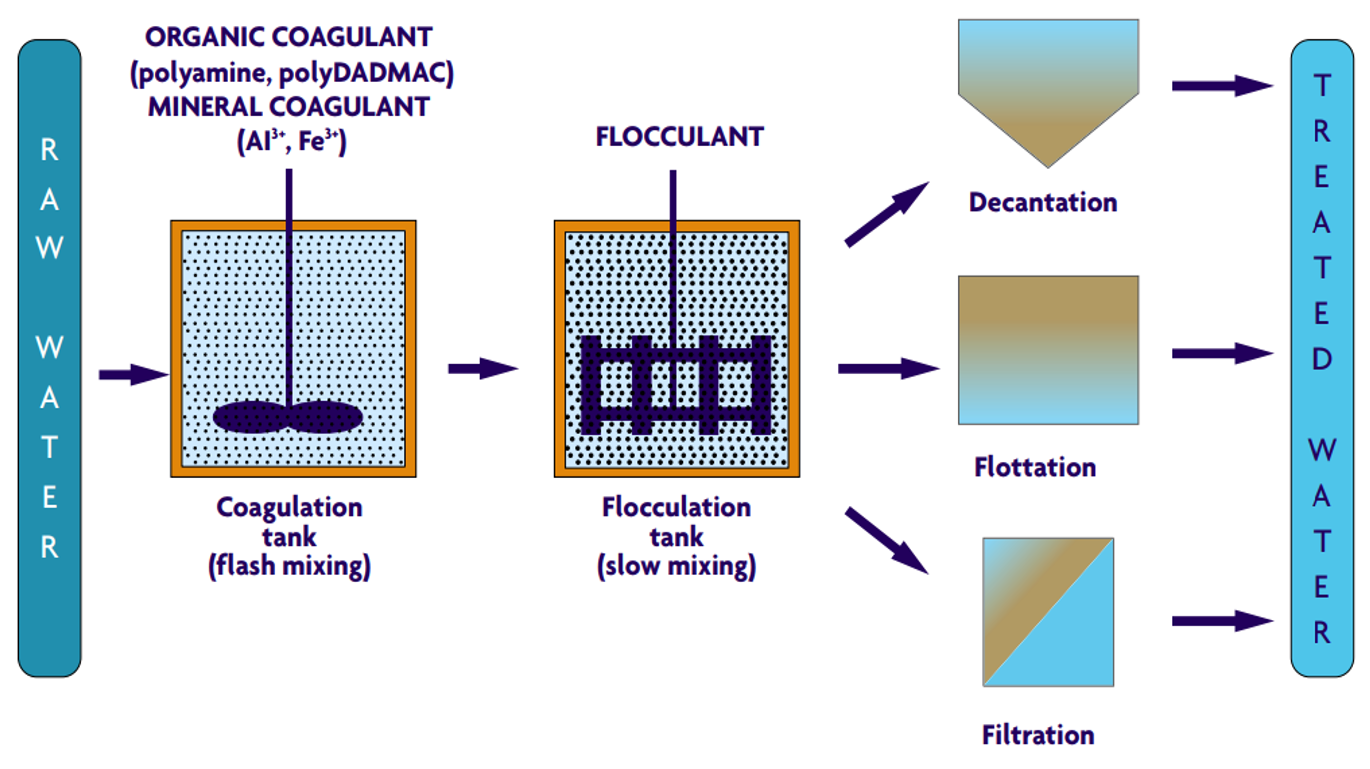 Coagulant Addition: Coagulants such as aluminum sulfate (alum), ferric chloride, or polymers are added to the water. The choice of coagulant depends on the specific characteristics of the water being treated and the desired quality of the treated water.
Rapid Mixing: The water is subjected to rapid mixing to ensure the even distribution of the coagulant throughout the water. This step is crucial for the effective neutralization of the charges on the suspended particles.
Flocculation: After rapid mixing, the water undergoes a slower mixing process known as flocculation. During this stage, the neutralized particles begin to aggregate into larger flocs. The mixing speed and duration are carefully controlled to promote the formation of flocs without breaking them apart.
Sedimentation: The water is then transferred to a sedimentation basin, where the flocs settle to the bottom due to gravity. The settled flocs form a sludge layer, which is periodically removed.
Filtration: The clarified water, now free of most suspended particles, passes through filters to remove any remaining impurities. Common filtration media include sand, gravel, and activated carbon.
Disinfection: Finally, the filtered water undergoes disinfection to eliminate any remaining pathogens. Chlorine, ozone, or ultraviolet light are commonly used disinfectants.
Coagulant Addition: Coagulants such as aluminum sulfate (alum), ferric chloride, or polymers are added to the water. The choice of coagulant depends on the specific characteristics of the water being treated and the desired quality of the treated water.
Rapid Mixing: The water is subjected to rapid mixing to ensure the even distribution of the coagulant throughout the water. This step is crucial for the effective neutralization of the charges on the suspended particles.
Flocculation: After rapid mixing, the water undergoes a slower mixing process known as flocculation. During this stage, the neutralized particles begin to aggregate into larger flocs. The mixing speed and duration are carefully controlled to promote the formation of flocs without breaking them apart.
Sedimentation: The water is then transferred to a sedimentation basin, where the flocs settle to the bottom due to gravity. The settled flocs form a sludge layer, which is periodically removed.
Filtration: The clarified water, now free of most suspended particles, passes through filters to remove any remaining impurities. Common filtration media include sand, gravel, and activated carbon.
Disinfection: Finally, the filtered water undergoes disinfection to eliminate any remaining pathogens. Chlorine, ozone, or ultraviolet light are commonly used disinfectants.
The scientific basis of chemical coagulation lies in the neutralization of electrical charges on suspended particles. Most particles in water carry a negative charge, causing them to repel each other and remain in suspension. Coagulants, which are typically positively charged, neutralize these negative charges, allowing the particles to come together and form larger aggregates.
Charge Neutralization: When coagulants are added to water, they dissociate into positively charged ions. These ions neutralize the negative charges on the suspended particles, reducing the repulsive forces between them.
Particle Aggregation: With the charges neutralized, the particles can come into close contact and aggregate. This aggregation is facilitated by the Brownian motion of particles, which causes them to collide and stick together.
Floc Formation: As the particles aggregate, they form larger and larger clusters, known as flocs. The size and strength of the flocs depend on factors such as the type and dose of the coagulant, the mixing conditions, and the characteristics of the water.
Sedimentation: The flocs, being larger and heavier than the individual particles, settle to the bottom of the sedimentation basin. The rate of sedimentation is influenced by the size and density of the flocs, as well as the viscosity of the water.
Application in Water Treatment

Chemical coagulation is widely used in both drinking water and wastewater treatment plants. Its primary purpose is to improve water quality by removing turbidity, color, and organic matter. The process also aids in the removal of pathogens and other contaminants, making the water safe for consumption or discharge.
Drinking Water Treatment: In drinking water treatment, coagulation is often the first step in the treatment process. It is used to remove natural organic matter, which can react with disinfectants to form harmful byproducts. By reducing turbidity, coagulation also enhances the effectiveness of subsequent disinfection steps.
Wastewater Treatment: In wastewater treatment, coagulation is used to remove suspended solids, organic matter, and nutrients such as phosphorus. This is particularly important in the treatment of industrial wastewater, where high concentrations of contaminants can pose significant environmental and health risks.
Enhanced Filtration: By removing a significant portion of suspended particles, coagulation reduces the load on filtration systems, extending their lifespan and improving their efficiency. This is especially beneficial in membrane filtration systems, where fouling can be a major issue.
Sludge Management: The sludge generated during the coagulation process can be further treated and disposed of or reused. In some cases, the sludge can be dewatered and used as a soil conditioner or in construction materials.
Improvement of Water Treatment Proceses
The incorporation of chemical coagulation into water treatment processes offers several advantages:
Improved Water Quality: Coagulation effectively removes a wide range of impurities, resulting in clearer, safer water. This is particularly important in drinking water treatment, where the removal of pathogens and organic matter is critical.
Cost-Effectiveness: By reducing the load on filtration and disinfection systems, coagulation can lower operational costs. The process also allows for the use of lower doses of disinfectants, reducing chemical costs and minimizing the formation of harmful byproducts.
Versatility: Coagulation can be adapted to treat different types of water, including surface water, groundwater, and industrial wastewater. The choice of coagulant and process parameters can be tailored to the specific needs of the treatment plant.
Environmental Benefits: By removing contaminants from wastewater, coagulation helps protect aquatic ecosystems and public health. The process also facilitates the recycling and reuse of treated water, contributing to sustainable water management practices.




.gif)
