Electrocoagulation (EC) is an advanced water treatment technology that utilizes electrical currents to remove contaminants from water. This method has gained prominence due to its effectiveness in treating various types of wastewater, including industrial effluents, municipal wastewater, and even drinking water. The process involves the use of sacrificial electrodes, typically made of aluminum or iron, which release metal ions into the water to facilitate the coagulation of suspended particles, emulsified oils, and other contaminants.
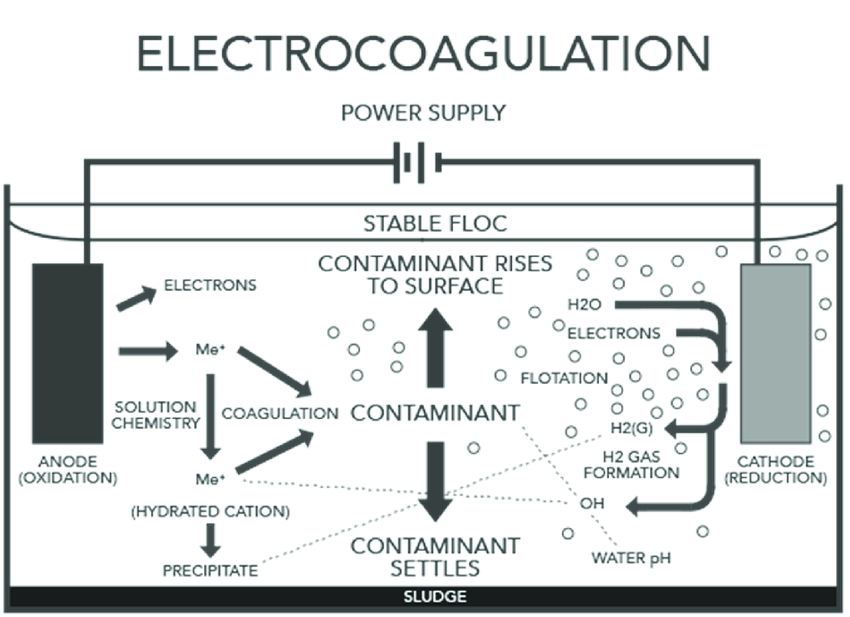
From an engineering standpoint, the electrocoagulation system comprises several key components and processes:
- Electrocoagulation Cell:
- The core of the system where electrochemical reactions occur.
- Contains electrodes immersed in water.
- Power Supply:
- Provides the necessary direct current (DC) to drive electrochemical reactions.
- Electrodes:
- They are typically made of sacrificial metals such as aluminum or iron.
- They are arranged in a parallel configuration to maximize contact area with water.
- Periodically cleaned or replaced to maintain efficiency.
- Process Initiation:
- Application of DC to the electrodes.
- The anode undergoes oxidation, releasing metal ions into the water.
- Cathode reduces water, producing hydrogen gas and hydroxyl ions.
- Coagulation & Flocculation
- Metal ions neutralize charges on suspended particles, forming larger flocs.
- Hydroxyl ions increase water pH, enhancing coagulation.
- Gas Flotation
- Hydrogen gas bubbles attach to flocs, causing them to float to the surface.
- Flocs are easily removed from the water surface.
- Design Considerations
- Type and concentration of contaminants.
- Flow rate of the water.
- Desired level of treatment.
- Control of power supply to optimize electrochemical reactions.
The design of the electrocoagulation system must consider several factors, including the type and concentration of contaminants, the flow rate of the water, and the desired level of treatment. The electrodes must be periodically cleaned or replaced to maintain their efficiency, and the power supply must be carefully controlled to optimize the electrochemical reactions.
The scientific principles underlying electrocoagulation are based on electrochemical reactions and the principles of coagulation and flocculation. When an electrical current is applied to the electrodes, several reactions occur simultaneously:
The overall efficiency of the electrocoagulation process depends on several factors, including the type and concentration of the metal ions, the pH of the water, the electrical current density, and the contact time between the electrodes and the water.

Application in Water Treatement
Electrocoagulation is used in various water treatment applications due to its versatility and effectiveness. Some of the key applications include:
Advantages of Electrocoagulation
Electrocoagulation offers several advantages over conventional water treatment methods:
- Industrial Wastewater Treatment: Electrocoagulation is widely used to treat industrial effluents containing heavy metals, oils, and other contaminants. The process can effectively remove these pollutants, making the treated water suitable for discharge or reuse.
- Municipal Wastewater Treatment: In municipal wastewater treatment plants, electrocoagulation is used to remove suspended solids, organic matter, and pathogens. The process can be integrated with other treatment methods, such as biological treatment and filtration, to enhance the overall efficiency of the treatment plant.
- Drinking Water Treatment: Electrocoagulation can be used to treat drinking water by removing turbidity, color, and pathogens. The process is particularly effective in removing contaminants that are difficult to treat with conventional methods, such as arsenic and fluoride.
- Oil and Gas Industry: In the oil and gas industry, electrocoagulation is used to treat produced water and other wastewater streams. The process can remove emulsified oils, suspended solids, and other contaminants, making the treated water suitable for reuse or discharge.


.gif)
